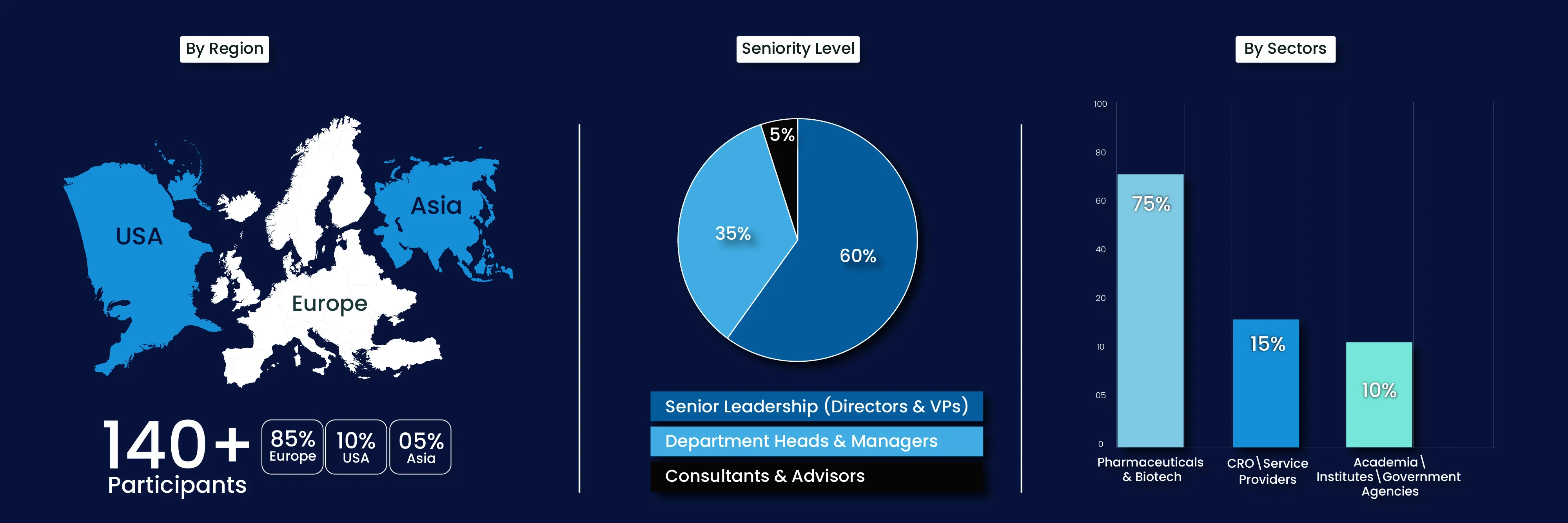
About CTS 2026
The Clinical Trial Supply Forum is a premier B2B platform uniting industry leaders, innovators, and stakeholders across the clinical supply chain. This two-day event is designed to address the complexities of clinical trial logistics, discuss emerging technologies, and share strategic solutions that drive efficiency, transparency, and compliance in global clinical supply operations.

We champion agile, tech-driven clinical supply that enhances patient access, sustainability, and speed. Our goal is to equip supply leaders with tools to cut risk, boost resilience, and accelerate therapy delivery.
We see clinical supply chains that are smart, digital, and patient-first—powered by real-time tracking, decentralized delivery, and sustainable practices. CTS 2026 is where strategy meets innovation in supply excellence.
in supply chain planning, patient-centric trials, and risk mitigation.
on temperature-controlled logistics, direct-to-patient models, and IRT integration.
on managing decentralized trials and overcoming import/export challenges.
with supply chain leaders, clinical trial sponsors, CROs, and technology vendors.
Meet the Global Community of Health Innovators